TEAM MEMBERS
Control and dynamics of the ovarian cycle
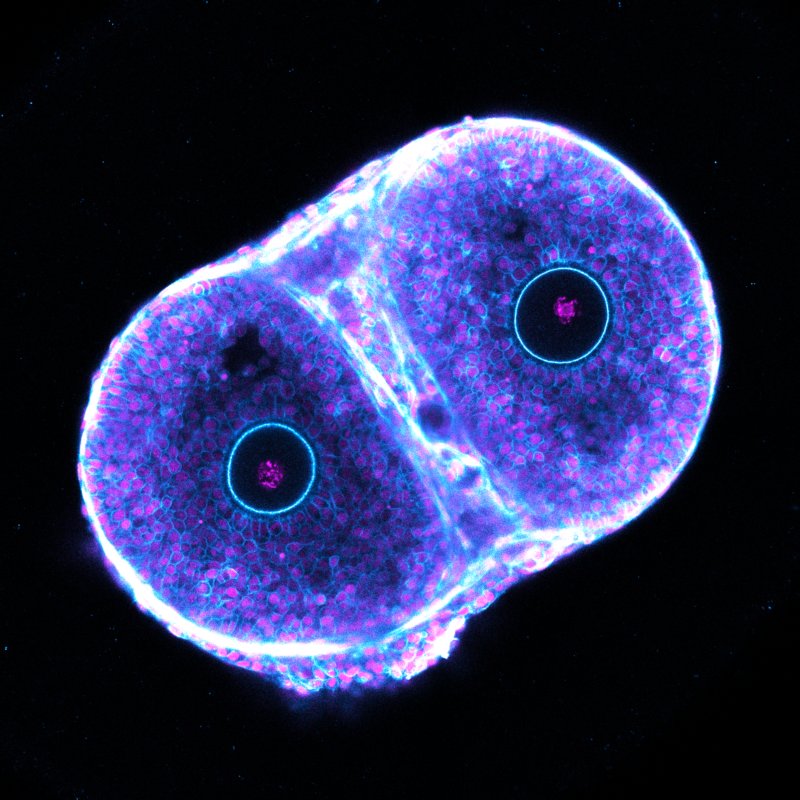
Our team uses ex vivo imaging strategies to model ovarian follicle growth, selection, and ovulation, investigating how cell-to-cell and follicle-to-follicle communication ensures the production of a fertilisable egg.
The origin of new life is one of the most fundamental questions of human existence. The maternal contribution to this new life, the egg, is released from an ovarian follicle during ovulation. Of the millions of follicles present in the ovary at birth, only a small fraction will ever be selected for ovulation, and most will degenerate. Even among those selected, many fail to ovulate or encounter errors during the first meiotic division of the egg, which occurs within the ovulating follicle. Large-scale follicle loss, ovulation failure, and meiotic errors are major causes of fertility decline with maternal age.
Female fertility is governed by communication between the egg and surrounding follicle cells, as well as competition between follicles within the ovary. Understanding how interactions at the cellular and tissue scales drive reproductive function requires a detailed picture of the cycling ovary. Yet, despite their fundamental nature and medical implications, how follicles grow, compete, and coordinate ovulation and meiosis has never been studied directly.
With colleagues in my previous lab, I developed a model to study ovarian processes live in isolated mouse follicles using a long-term culture system with advanced quantitative imaging. This laid the foundation for groundbreaking work in which we visualised and described the mechanism of mammalian ovulation (Thomas, Marx et al., 2024). This model provides an unprecedented view into the dynamics of the cycling ovary, allowing us to address fundamental questions about fertility that were previously inaccessible.
At the IBDM, my team builds on this approach, developing ex vivo strategies to uncover how follicles compete for selection and how communication between the oocyte and follicle cells ensures proper coordination of ovulation and meiosis. Our work tests the hypothesis that communication at both the cell-to-cell and follicle-to-follicle scales is essential for producing a fertilisable egg.
Publications
Ex vivo imaging reveals the spatiotemporal control of ovulation.
Ex vivo imaging reveals the spatiotemporal control of ovulation.
News
The IBDM welcomes three new research groups!
We would like to extend a warm welcome to Julia Schaeffer, Baptiste Libé-Philippot and Christopher Thomas.
No jobs opportunities found..