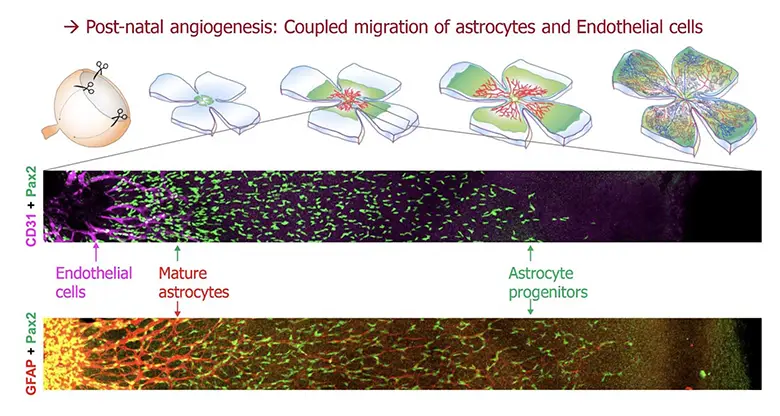
Endothelial cells (ECs) are the building blocks of blood vessels, which assemble and grow through a process called angiogenesis. In the retina, angiogenesis is driven by a cross-talk between ECs and local cell types such as astrocytes or neurons. Retinal ECs form vessels during the first postnatal week, by migrating on a carpet of astrocytes, which migrate ahead of them, in a radially polarized direction (from the center to the periphery). The two migration events are coupled by known mechanisms involving patterned regulation of morphogenetic signals produced by astrocyte and endothelial cells. Thus, any alteration of astrocyte migration also interferes with angiogenic progression in the retina.
The Helmbacher team identified the Fat1 Cadherin as a novel regulator of retinal vascular integrity, and investigated the underlying mechanisms. A new study published in Development describes how a combination of astrocyte-intrinsic and extrinsic Fat1 activities (deciphered owing to tissue-specific and inducible genetic tools) influences retinal vascular development by modulating astrocyte migration polarity, proliferation, and maturation.
To know more



